IMPACT
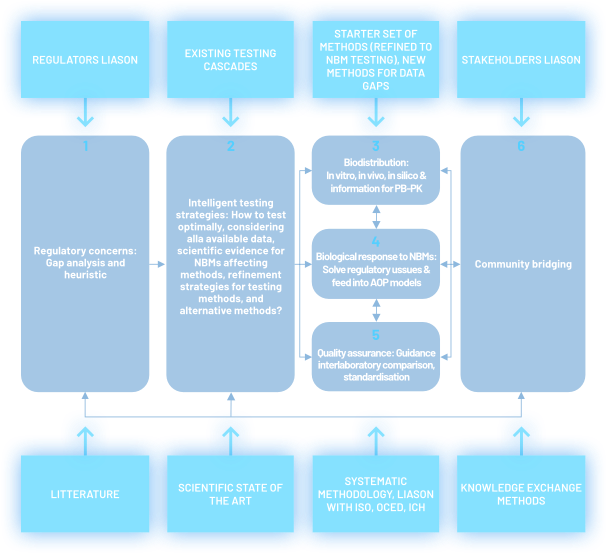
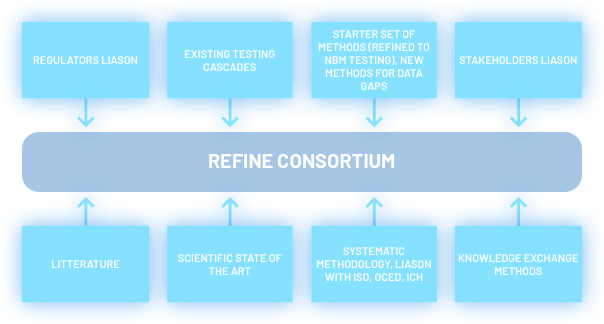
- REFINE will help to establish a European Consortium for the Advancement of Regulatory Science in Biomaterials and Nanomedicines with representation from all the stakeholder communities.
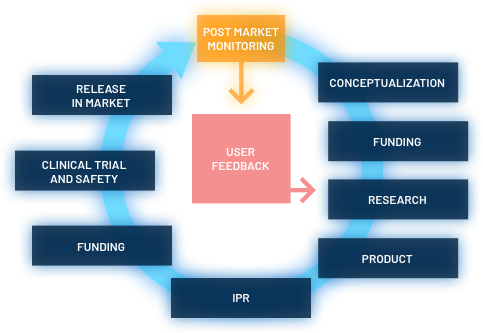
- REFINE will identify critical issues for innovative products and establishment of an action plan for further studies.
- REFINE will deepen existing and establish with existing European Infrastructures along with relevant European Research Networks;
- Within REFINE we will elaborate an action plan for a better integration of the European Union with other regions of the world.
- REFINE will help to reduce the cost of preclinical and clinical development of NBMs.
- REFINE will help to reduce the time for innovations to reach the patients.
- REFINE will provide tools for more informed risk assessment and decision-making.
- REFINE will help to improve standardisation of regulatory practice at the European and international level.
- REFINE will foster close collaboration with the stakeholders communities.
REFINE literature in Regulatory Science
Key Deliverables
White paper describing the refinement of standard testing methods
Reflection document describing new methods developed to further predict the biological behaviour of new nanomedicines and nanodevices
Reflection document correlating in vitro to in vivo methodologies
Milestones
Knowledge Exchange Conferences
Bridging community and broad dissemination engagement
Dissemination of documents across the stakeholders